
QUALITY OF LIFE
A wellness wellspring, FOCUS hemp extract is proven to reduce perceived stress, enhance sleep patterns, and boost basic and complex cognitive processes, including attention, memory, and concentration.

UNLOCK YOUR BRAIN'S POTENTIAL
Using advanced neurofeedback brain mapping technology, our medical advisory team screened more than 100 subjects on the effects FOCUS has on quality of life and brain and psychological health. The results? Clinically validated wellness improvements that are changing lives. Boost your quality of life & unlock your wellness potential with FOCUS! See the results of FOCUS' Quality Of Life Improvement Screenings below.

THE FOCUS EFFECT ON QUALITY OF LIFE
Young or old, male or female, FOCUS is statistically proven to enhance general wellness and quality of life. Over the course of a three-month span, 121 screening participants underwent routine screenings on the effects FOCUS has on a number of quality of life indicators, including mental stamina, physiological health, and mind-based markers like concentration, comprehension, and memory.
To monitor participants’ progress we utilized their responses and scores to Medical Symptom/Toxicity Questionnaires (MSQ). The MSQ is a clinical standard questionnaire used to identify and assess a variety of quality of life factors. Comparing screening results to an initial quality of life baseline score of 59.8 (out of 100 points), after three months of daily use of FOCUS, screening participants’ quality of life scores improved by 63% to an average MSQ score of 37.8. *A descending MSQ point score is evidence of a quality of life improvement.
The Effects Of FOCUS Broad Spectrum Hemp Extract On MSQ
Objectives/Hypothesis
FOCUS is able to improve the quality of life of its users, regardless of gender over a three-month span.
Subject Base & Administration Methods
Enrolled Subjects: 121 (62 female 59 male)
Completed Observations: 121
Upon a chart review, we looked at 121 patients who were instructed to use FOCUS. We looked at the Medical Symptom Questionnaire (MSQ) at baseline, then provided them with a follow up MSQ at months one, two, and three. Patients were instructed to use 1mL of FOCUS once per day in the morning. The reason we chose to use the MSQ is because it is the most reliable quality of life scoring system. This questionnaire is used by integrative care centers, such as The Cleveland Clinic Center for Functional Medicine, to help identify symptoms across a broad range of categories so that it is easily tracked. Perceived symptoms are divided into specific categories such as digestion, head, pain, heart, mind, emotions, and others.
Results/Outcome
There are statistically significant differences between the use of FOCUS starting at time zero to month one, month two, and month three, all with p value of 0.000. Multivariate tests were used for cross comparison for statistical significance. When analyzing gender differences, there is no statistical significance (p value of 0.493), therefore gender has no influence on the change of MSQ scores over the three-month collection time. The initial starting MSQ score is a mean of 59.8. This is improved by 20.4 points after one month, which translates to an improvement of 34%. After two months, the mean MSQ difference is a decrease of 30.1, which translates to an improvement of 50%. After three months, the mean MSQ difference decreased to 37.8, which is an improvement of 63.2%.
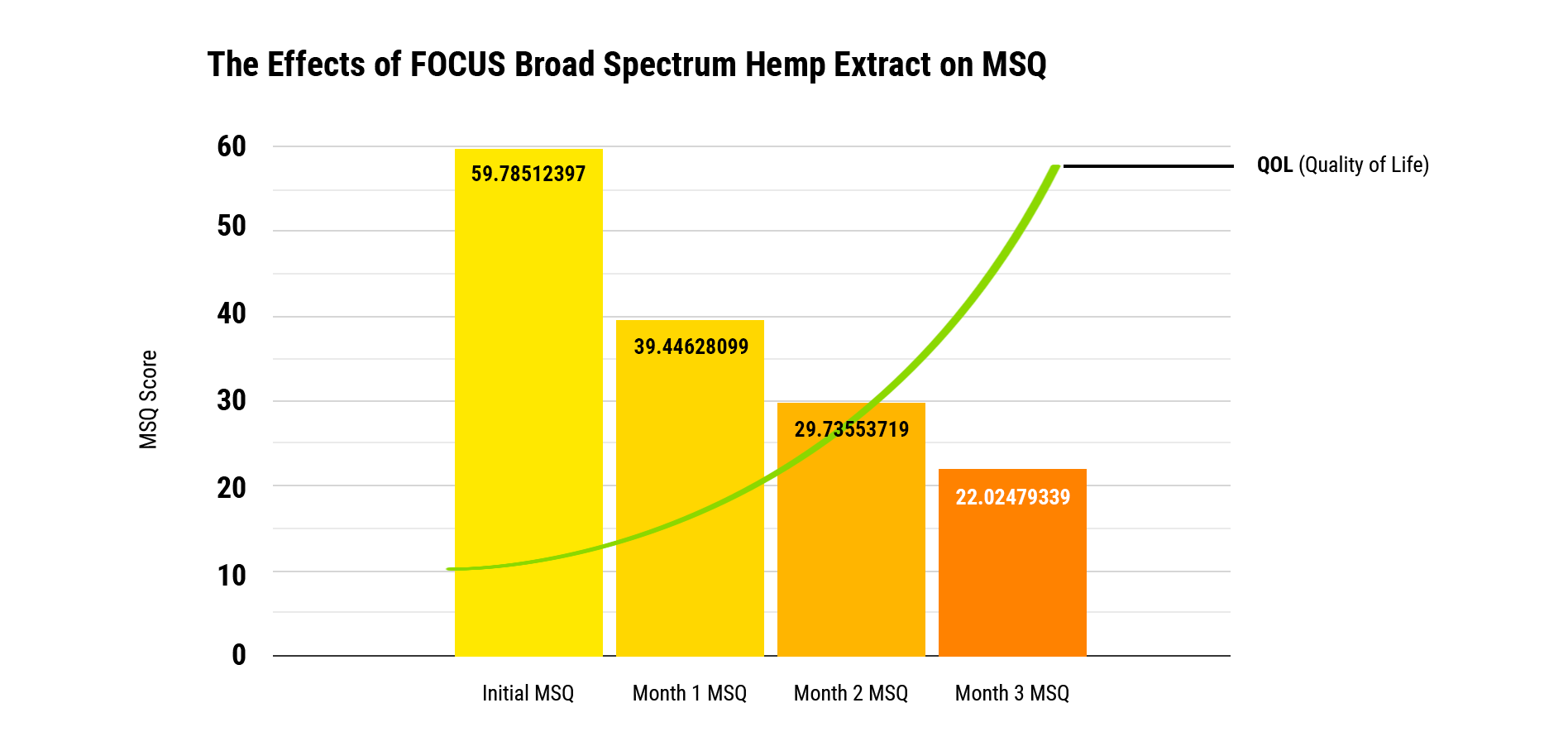
Conclusion
Using FOCUS can provide a significant level of improvement in quality of life for at least three months when used consistently at 1 mL per day. This is encouraging data since we find statistical differences in each month of improvement, regardless of gender. The value in knowing the quality of life data is that these results provide us with a solidified explanation of how much FOCUS can improve quality of life over three months with daily use.
An overall improvement in quality of life (63.2%) is a remarkable difference even when compared to traditional wellness protocols like lifestyle modifications, mindfulness, and other mental exercises. This provides those who use FOCUS with fantastic opportunities to help improve their quality of life in as short as a three-month span.

THE FOCUS EFFECT ON PERCEIVED STRESS
Whether stemming from personal, professional, or emotional factors, every person on the planet deals with varying levels of stress on a daily basis. How you manage that stress has a direct effect on short- and long-term health.
After screening 121 participants over a three-month span, results conclude that a daily regimen of FOCUS reduces personal stress levels—leading to a healthier quality of life and more joyful psychological wellness. To assess FOCUS’ effect on perceived stress our medical advisory team utilized periodic participant responses to a classical psychological and stress assessment tool known as the Perceived Stress Scale (PSS).
Comparing screening results to a PSS baseline of 25.7 (out of 100 points), after three months of daily use of FOCUS, screening participants’ PSS scores improved upwards of 75% to 8.12. *A descending PSS point score is evidence of perceived stress improvement.
The Effects Of FOCUS Broad Spectrum Hemp Extract On Perceived Stress Scale
Objectives/Hypothesis
FOCUS is able to improve stress levels.
Subject Base & Administration Methods
Enrolled Subjects: 121 (62 female 59 male)
Completed Observations: 121
To analyze improvements in stress, we used the Perceived Stress Scale (PSS). The Perceived Stress Scale (PSS) is the most widely used psychological tool for measuring the perception of stress. It is useful to measure perceived stress levels during common life events. Furthermore, this scoring system is a standard of care in the analysis of stress. Upon a chart review, we looked at 121 patients who were instructed to use FOCUS. We looked at the PSS at baseline, then provided them with follow up PSS at months one, two, and three. Patients were instructed to use FOCUS at 1 mL once per day in the morning. All collection of data was via telephone with verbal instructions.
Results/Outcome
The initial PSS score is 25.7 with a standard deviation of 11.8. After one month the average PSS improved to 15.6 with a standard deviation of 9.6, which translates to an improvement of 39%. In month two, the average score improved to 11.6 with a standard deviation of 8.3, translating to an improvement of 55% compared to baseline. By month three, the mean score improved to 8.12 with a standard deviation of 6.3, translating to a mean improvement of 75%. All values were statistically significant according to multivariate tests for comparison.
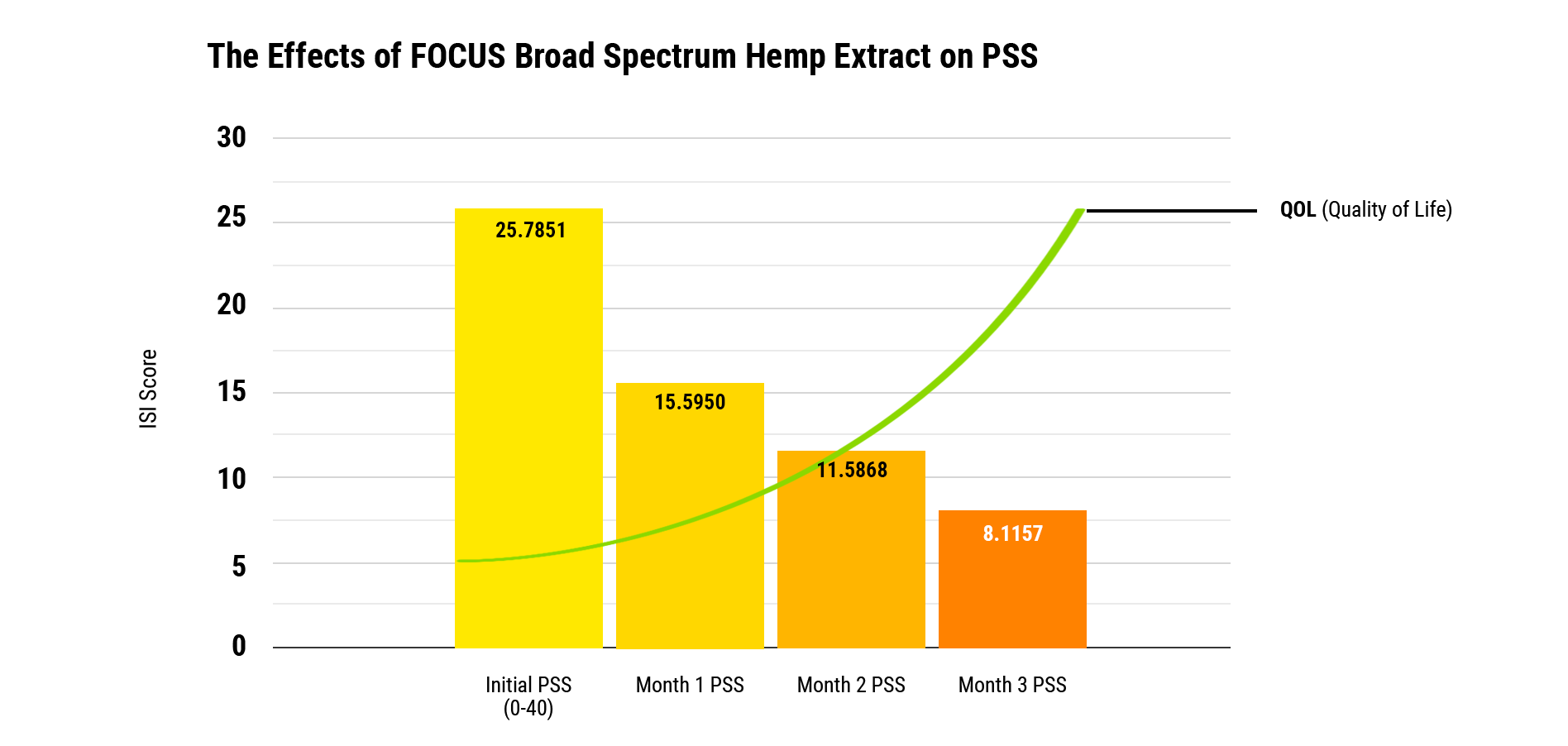
Conclusion
Perceived stress is an important driver of impaired quality of life. Using the Perceived Stress Scale, we are able to quantify stress level improvements after using FOCUS over a three-month period. Scores were significantly improved compared to baseline after each month. Analyzing this data, we are able to gather tremendous insight into how much stress can improve. This provides those who use FOCUS daily with fantastic opportunities to help improve their stress levels and their quality of life.

THE FOCUS EFFECT ON SLEEP PATTERNS
Sleep deprivation and poor sleep cycles can take a toll on body function and general wellness, and can potentially lead to serious psychological and physiological ailments.
Following a three-month, 121 participant sleep-based screening, FOCUS’ blend of hemp compounds and botanicals is now confirmed to help balance sleep patterns and circadian rhythm. To gauge FOCUS’ impact on sleep pattern improvement each participant periodically completed an Insomnia Severity Index (ISS) questionnaire. ISI is the most widely used assessment instrument in clinical and observational studies of insomnia and sleep issues.
Comparing screening results to a ISI mean baseline of 18.7 (out of 100 points), after three months of daily use of FOCUS, screening participants’ ISI scores improved to 5.33—a percentage improvement of more than 71%. *A descending ISI point score is evidence of a sleep improvement.
The FOCUS Effect On Sleep Patterns
Objectives/Hypothesis
Over a three-month span, FOCUS is able to improve the quality of sleep in its users, regardless of gender.
Subject Base & Administration Methods
Enrolled Subjects: 121 (62 female 59 male)
Completed Observations: 121
To analyze improvements in sleep, we used the Insomnia Severity Index. The Insomnia Severity Index (ISI) is a brief screening assessment tool designed to evaluate sleep problems. It was developed by Charles M. Morin, Ph.D., Professor of Psychology at the Université Laval in Quebec City, Canada and a world leading authority on insomnia and its treatment. The ISI is one of the most widely used assessment instruments in clinical and observational studies of insomnia.This index is a quantifiable measurement that has been utilized widely as a tool for insomnia measurement. Furthermore, this scoring system is a standard of care in the analysis of sleep. The ISI is a classical assessment tool used in neurology as a standard of care in monitoring sleep issues. Upon a chart review, we looked at 121 patients who were instructed to use FOCUS daily. We looked at the ISI scores at baseline, then provided patients with follow up ISI questionnaires at months one, two, and three. Patients were instructed to use FOCUS at 1 mL once per day in the morning. All collection of data was recorded via telephone with verbal instructions.
Results/Outcome
ISI scores had statistically significant decreases over the course of three months compared to the baseline. Insomnia is shown to significantly decrease over the analyzed period of three months. The mean starting score for the ISI is 18.7 with a standard deviation of 6.76, which is an improvement by 40% just after using for one month with a score of 11.28 with a standard deviation of 5.83. On month two, the ISI improved by 47% compared to baseline. On month three, scores continue to improve by 52% to an average score of 8.8 with a standard deviation of 5.0. On month three, the ISI improved by 71% with a mean score of 5.33 with a standard deviation of 4.37. All values were statistically significant according to multivariate tests for comparison.
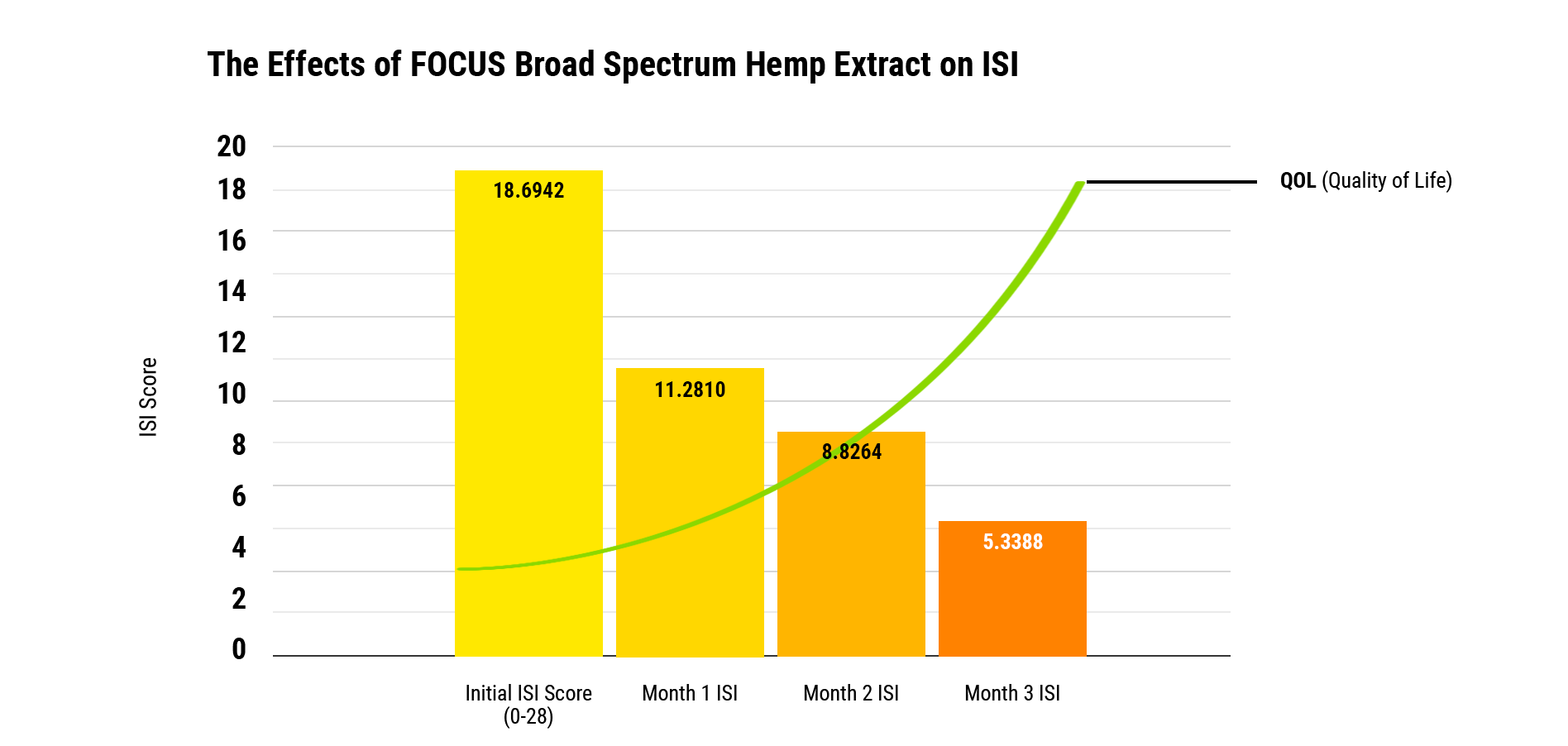
Conclusion
Using FOCUS daily, subjects showed an impressive improvement in sleep over the course of three-month screening. This shows that FOCUS is able to consistently improve sleep over a three-month period when used consistently at 1 mL per day. This is encouraging data since we find large improvements in sleep in each month of improvements, regardless of gender. This sleep data provides us with valuable, evidence-based reasonings of how much FOCUS can improve sleep. Utilizing this data, we are able to collect tremendous insight about how much sleep can improve. This provides those who use FOCUS with fantastic opportunities to help improve their sleep consistently.
